American History
Explore the development of the United States with this collection of articles about American history. Topics in this section include the American Revolution, the gold rush and the expansion of the West.

How the Great Compromise Saved a Fledgling United States
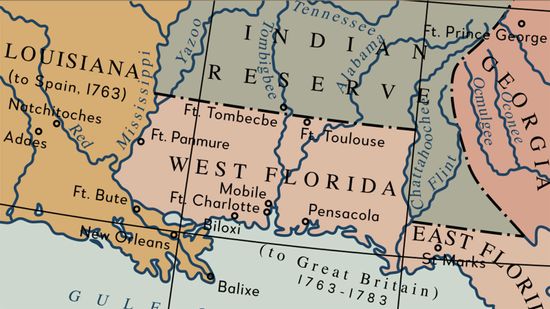
These 6 U.S. States Once Declared Themselves Independent Nations

Bacon's Rebellion: America's First Armed Insurrection

Harpers Ferry Has a Complex and Dizzying History

The Racial History of the Term 'Grandfathered In'

Slavery Under Another Name: What Were the Black Codes?

A Roundup of Some of the Wildest Characters in the Wild West

Reign of Terror: The Forgotten Story of the Osage Tribe Murders

Which Native American Nations Were the 'Five Civilized Tribes'?

SS United States: Dock in Disrepair or Sink as Reef Fodder?

Stardust and Scandal: The Hollywood Sign Turns 100

5 Events in Black History You Never Learned in School

Why in the World Do U.S. Presidents Pardon Turkeys?

The Truman Doctrine Transformed U.S. Foreign Policy Forever

Ridiculous History: H-Bombs in Space Caused Light Shows, and People Partied

How the Harlem Renaissance Sparked a New African American Identity

5 Causes of the Great Depression: Could It Happen Again?

What's Inside Mount Rushmore's Not-So-Secret Chamber?

10 Least Diverse States in the U.S.

14 Least Visited National Parks in the United States

Is Molossia a Real Country? Well, It's Not a Sovereign Nation
Learn More
Castles aren't just a European thing. You can find impressive stone towers, dramatic spires, and fascinating stories scattered across the Buckeye State.
If you're looking for diversity in the United States, you're not alone. Researchers, policymakers and businesses alike want to understand how populations vary in terms of race, religion, culture, language, education and more.
Some people visit museums for history. Others prefer something a little spookier. The most haunted places in America mix eerie ambiance with tragic backstories and alleged ghostly encounters.
Advertisement
Most people flock to Yellowstone, Yosemite, or the Grand Canyon, but the least visited national parks offer something just as remarkable: solitude.
The flag of Texas, known proudly as the Lone Star Flag, is one of the most recognizable symbols in the United States. Often associated with Texas pride and independence, the flag design reflects the state’s rich history and lasting identity as the Lone Star State.
From ancient kingdoms to pivotal moments in global history, the Hawaii landmarks scattered across the Rainbow State offer visitors a journey through centuries of Native Hawaiian culture, national significance and natural wonder. Here's what to explore on the major Hawaiian islands.
Everything's bigger in Texas, including its history. From pre-Columbian archaeological wonders to grand battlegrounds, the Lone Star State packs a punch when it comes to heritage. Texas landmarks aren't just tourist stops; they're touchstones to defining moments in both Texas history and national history.
Advertisement
From rugged coastlines to iconic bridges, California landmarks stand as monumental reminders of the Golden State's deep and diverse history. Whether you're hiking the redwoods of Northern California or exploring sunbaked missions in Southern California, there's a landmark with a story waiting to be told.
Chicago is packed with some of the most celebrated sights in the United States. From the glimmering lakefront to the towering skyscrapers, Chicago landmarks tell a vibrant story of architecture, innovation and culture that you can experience all in one epic city.
Ever wondered why certain places in the U.S. get all the postcards and bucket list glory? They're often U.S. landmarks that pack a punch with history, culture and awe-inspiring views. These aren't just photo ops; they're vivid chapters in the story of American history, woven into the landscape from sea to shining sea.
Once the crown jewel of American maritime innovation, the SS United States is again making waves — but this time, it might be under the sea.
Advertisement
Nestled in the Nevada desert lies one of the world’s most curious micronations: the Republic of Molossia.
The term "commonwealth states" refers to a specific political status that four states in the United States use. These states retain the same powers and privileges as all other U.S. states, but their choice to use commonwealth reflects their history, values and governance.
By Yara Simón
The Southern states include both sprawling farmlands and bustling cities. Often called "the South," this area played a pivotal role in shaping the identity of the United States.
By Yara Simón
The Midwest region, also known as the Middle West or the North Central Region of the United States, is home to Lake of the Ozarks, the National Underground Railroad Freedom Center and Mall of America. With the agriculture and manufacturing industries, the area has also had a big economic impact.
By Yara Simón
Advertisement
New England is one of the most historic and scenic regions of the United States. Nestled in the northeastern corner of the country, the New England region played an important role in the founding of the United States.
By Yara Simón
The Tuskegee syphilis study, also known as the U.S. Public Health Service (USPHS) Untreated Syphilis Study at Tuskegee, is one of the most infamous chapters in the history of U.S. public health and scientific research. PHS, in collaboration with the Tuskegee Institute, conducted the study, which aimed to observe the natural history of untreated syphilis in Black men from Macon County, Alabama.
By Yara Simón
California, the Golden State, is synonymous with stunning beaches, innovative tech hubs and Hollywood glamour. As the most populous state in the U.S., California is home to some of the nation's largest and most influential cities.
By Mack Hayden
The least populated state in the U.S. offer a unique glimpse into the country's quieter and slower ways of life.
By Ada Tseng
Advertisement
The 13 original colonies of the United States were the foundation of what would become a new nation, born from a blend of ambition, conflict and compromise. These colonies stretched along the Atlantic coast, from New Hampshire in the north to Georgia in the south.
By Mack Hayden
Few things capture the spirit of adventure quite like the vast network of highways stretching across the United States. But have you ever wondered which road takes the crown as the longest?
By Mack Hayden
From time to time, the United States federal government experiences a shutdown, reminding the country's citizens just how difficult it is to align federal funding with political power and public policy.
By Marie Look
Off the top of your head, you may know JFK as the youngest president in U.S. history, and you'd be correct that he was the youngest to be elected. But what about the youngest person to ascend - that is, the youngest VP to become president after the death of an incumbent?
By Mitch Ryan
Advertisement
President Joe Biden became the oldest president in U.S. history when he took the oath of office in 2020 at 78 years old. The former senator of Delaware also served as vice president for two consecutive terms of the Obama administration between 2009 and 2017.
By Mitch Ryan
State capitals provide a centralized location for the state's administrative, legislative and sometimes judicial branches. This facilitates efficient governance by bringing together key decision-makers, lawmakers and administrative staff in one place, streamlining communication, coordination and policy implementation.
By Karina Ryan
























